Comparison of the intraarticular injection of platelet rich plasma (PRGF®) and hyaluronic acid (Hyalone®) in the treatment of chondral lesions: a randomized, prospective clinical study
Comparación de la inyección intraarticular de plasma rico en plaquetas (PRGF®) y ácido hialurónico (Hyalone®) en el tratamiento de las lesiones condrales: estudio clínico prospectivo aleatorizado
Resumen:
Objetivo: comparar el efecto de la inyección intraarticular de plasma rico en plaquetas (PRGF®) y ácido hialurónico (Hyalone®) en el tratamiento de las lesiones degenerativas del cartílago de la rodilla.
Métodos: estudio prospectivo, aleatorizado y abierto, que compara el efecto clínico del tratamiento con PRP (PRGF®) y ácido hialurónico (Hyalone®) en pacientes con lesiones condrales degenerativas (no traumáticas) de rodilla. Se incluyeron 80 pacientes y se aleatorizaron en 2 grupos de 40 pacientes cada uno. La valoración clínica se realizó inicialmente y a los 6 meses del tratamiento mediante las escalas Knee Injury and Osteoarthritis Outcome Score (KOOS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) y la escala visual analógica (EVA).
Resultados: en ambos grupos se produjeron mejorías significativas respecto de los valores basales. Los pacientes tratados con PRP mejoraron en la valoración del dolor por la EVA con respecto al grupo AH, mostrando unas mejorías de 2,08 (1,5) y 0,47 (1,7), respectivamente (PRGF® vs. Hyalone®; p = 0,001). Sin embargo, no se apreciaron diferencias entre ambos grupos tanto en el WOMAC como en la escala KOOS (p > 0,05).
Conclusiones: la inyección intraarticular de PRP no mostró una mejoría clínica consistente respecto al tratamiento con AH a los 6 meses de seguimiento. Aunque se observaron diferencias favorables en la EVA, estas no se tradujeron en el resto de las escalas evaluadas.
Nivel de evidencia: IV.
Abstract:
Objective: To compare the effect of the intraarticular injection of platelet rich plasma (PRGF®) and hyaluronic acid (Hyalone®) in the treatment of degenerative cartilage lesions of the knee.
Methods: A randomized, prospective open-label study was made to compare the clinical effect of treatment with platelet rich plasma (PRP) (PRGF®) and hyaluronic acid (Hyalone®) in patients with degenerative (not traumatic) chondral lesions of the knee. A total of 80 patients were randomized to two groups of 40 patients each. Clinical assessment was made initially and 6 months after treatment using the Knee Injury and Osteoarthritis Outcome Score (KOOS), the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and a visual analogue scale (VAS).
Results: Both groups showed significant improvements with respect to the baseline values. The patients treated with PRP showed greater pain reduction than those treated with hyaluronic acid, according to the VAS, with improvements of 2.08 (1.5) and 0.47 (1.7), respectively (PRGF® versus Hyalone®; p = 0.001). However, neither the WOMAC nor the KOOS showed differences between the two groups (p > 0.05).
Conclusions: The intraarticular injection of PRP did not result in consistent greater clinical improvement versus hyaluronic acid after 6 months of follow-up. Although differences favourable to PRP were recorded with the VAS, they were not confirmed by the rest of the scales used.
Level of evidence: IV.
Introduction
Chondral and osteochondral lesions are a common problem in routine orthopedic surgical practice. These lesions can progress towards symptomatic osteoarthrosis, often affecting patient activities of daily living(1,2).
Many both surgical and non-surgical treatments are used in routine clinical practice, including microfractures, autologous chondrocyte transplantation and allogenic and autologous osteochondral grafts. The clinical outcomes of these techniques vary, and they are unable to guarantee that the degenerative process will be stopped over the long term(3). Avoiding progression to osteoarthrosis and affording pain relief without surgery are the main goals of biological treatments. At present, total knee replacement surgery is the ultimate treatment option for advanced osteoarthrosis(4).
There has been growing interest in the use of platelet-rich plasma (PRP) in recent decades. Preclinical studies in animals have demonstrated its potential usefulness, stimulating chondrogenesis and extracellular matrix recovery - this in a certain way suggesting a regenerative effect(5,6,7,8). Despite this preclinical and theoretical basis for the use of PRP in the treatment of cartilage lesions, there is still controversy regarding its in vivo clinical efficacy. In the year 2013, the Spanish Medicines Agency classified PRP as a "medicinal product for human use", encouraging investigators to precisely establish its indications, dosage and form of use in order to administer such biological treatment through clinical trials addressing each of the disease conditions and types of PRP. The use of the different types of PRP has become widespread in routine clinical practice, without the prior legal requirement to conduct clinical trials to assess their safety, viability or efficacy. This is because they were not conditioned by the regulatory aspects referred to medicinal products manufactured industrially or to advanced therapy medications, such as cultured mesenchymal stem cells, which are required by the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA) to abide by the Good Manufacturing Practice (GMP) guidelines(9). At present, due to the multiple procedures involved (open or closed, with or without leukocytes, with greater or lesser platelet content), the form of application, and the few clinical trials published to date, no clear conclusions can be drawn regarding the use of PRP(10,11,12).
The present study was carried out to compare the clinical effect of the intraarticular injection of PRP (PRGF®) and hyaluronic acid (HA, Hyalone®) in the treatment of degenerative cartilage lesions of the knee.
Material and Methods
A randomized (sequential, 1:1 proportion) prospective open-label study was carried out between February 2016 and January 2017 to compare the clinical effect of treatment with PRP (PRGF®) and HA (Hyalone®) in patients with degenerative (not traumatic) chondral lesions of the knee. A total of 80 patients were included in the study. All patients were duly informed and gave their consent for inclusion in the study. The included patients were diagnosed with chondral disease in the internal compartment of the knee according to the classification of the International Cartilage Regeneration and Joint Preservation Society (ICRS) (grade 2-4 with magnetic resonance imaging [MRI])(13).
Inclusion and exclusion criteria
The patients were included in the study based on the following inclusion and exclusion criteria. Inclusion criteria: patients of either gender, between 50-80 years of age, with a joint pain score of 2.5 points or more on the visual analogue scale (VAS), grade 2-4 radiological involvement according to the ICRS scale, and a body mass index (BMI) of 20-35 kg/m2.
Exclusion criteria: bilateral osteoarthrosis of the knee, requiring treatment of both knees; a prior diagnosis of polyarticular disease; severe mechanical deformity (varus / valgus of 15º); arthroscopy of the same knee in the 6 months before inclusion in the study; infiltration of the same knee in the 6 months before inclusion in the study; autoimmune or rheumatic disease; blood dyscrasias; corticosteroid therapy in the three months before inclusion in the study; use of nonsteroidal antiinflammatory drugs (NSAIDs) in the 15 days prior to inclusion in the trial; and intolerance to HA or avian proteins.
Patients receiving any kind of infiltration therapy of the knee or subjected to surgery of some kind were also excluded.
Treatment groups
The patients were randomized to two treatment groups. The PRP group (PRGF®) (n = 40) received a weekly intraarticular dose of PRP (PRGF®) during three weeks. The HA group (n = 40) received a single intraarticular dose of HA (Hyalone®).
Calculation of sample size
A sample size of 60 patients (30 subjects per group) was estimated in order to secure a statistical power of 95% in detecting a Cohen effect size of 1, assuming a standard deviation (SD) for both groups of 10 with an alpha value of 0.05 (two-tailed), and assuming a 10% patient loss rate. We decided to include 40 patients per group in order to compensate the possible negative impact of losses to follow-up.
Interventions and preparation of platelet rich plasma (PRGF®-Endoret®)
The medication was administered as an external para-patellar injection by surgeons exclusively dedicated to knee surgery. The patients in both groups were advised to avoid impact sports activities during the four weeks after injection of the first treatment dose in the PRP group and the single dose in the HA group. No restrictions referred to activity were applied after this period.
Autologous PRP was obtained from peripheral blood. The extracted sample was centrifuged in the BTI system (PRGF®-Endoret®, BTI Biotechnology Institute, Vitoria-Gasteiz, Spain) at room temperature (580 g, 5 min). Immediately prior to injection, the platelets were activated by adding 10% calcium chloride (0.05 ml of calcium chloride per ml of PRP).
The high molecular weight HA used was Hyalone® (60 mg/4 ml, Bioibérica, Barcelona, Spain), with application according to the instructions of the manufacturer.
Clinical follow-up
The patients were followed-up on for 6 months after infiltration. During this time, the assessment scales were sent by mail (conventional or e-mail, according to preference), since many of the patients lived outside the city of Pamplona and were not able to report to our centre in the specified time. Use was made of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)(14), the Knee Injury and Osteoarthritis Outcome Score (KOOS)(15) and the visual analogue scale (VAS)(16). These scales were assessed at the baseline (pre-treatment) visit and at the end of follow-up (6 months post-treatment).
Statistical analysis
Normal data distribution was assessed using the Kolmogorov Smirnov test. Comparison of the variables was made with the Student t-test for quantitative variables (paired or otherwise, as applicable) and the chi-square test for qualitative variables. The Stata 14 package (StataCorp 2015, Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP) was used for analysis of the data. The results were expressed as the mean (standard deviation [SD]).
Results
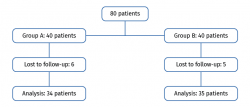
A total of 80 patients were randomized (sequential, 1:1 proportion) to two groups of 40 patients each. We excluded 6 patients in the PRP group (4 failed to answer the questionnaires and 2 were refractory to therapy) and 5 in the HA group (3 failed to answer the questionnaires and 2 were refractory to therapy). Of the patients regarded as refractory to therapy, one in the PRP group underwent total knee replacement surgery. Of the two patients in the HA group, one underwent total knee replacement surgery and the other received corticosteroid-anesthetic infiltration. A total of 34 patients were finally evaluated after 6 months in the PRP group, versus 35 patients in the HA group (Figure 1). The demographic data are reported in Table 1. There were no significant differences between the groups at baseline (age, gender, ICRS, WOMAC, KOOS and VAS); the groups were therefore considered to be comparable.
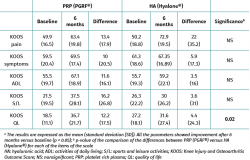
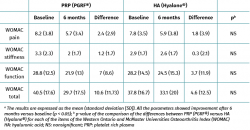
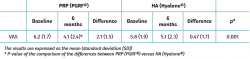
Both groups showed improvements in all the analysed clinical variables with respect to baseline (Tables 2 to 4). In relation to the three study scales, on comparing the two treatment groups only the VAS score was seen to differ, with results favourable to PRP (PRGF®), which showed an improvement of 2.1 (1.5) points with respect to baseline versus 0.47 (1.7) points in the HA group (Hyalone®) (p = 0.001) (Table 2). No differences were observed between the two groups in terms of the items assessed by the KOOS, with the exception of quality of life, where the results were favourable to treatment with PRP (PRGF®) (p = 0.02) (Table 3). Likewise, no significant differences were observed between the two groups in terms of the WOMAC, despite the fact that the magnitudes obtained were favourable to treatment with PRP (PRGF®) (Table 4).
Discussion
The intraarticular injection of PRP (PRGF®) resulted in greater improvement according to the VAS score than HA treatment (Hyalone®), although the rest of the scales (KOOS and WOMAC) evidenced no comparative improvement in function, pain or leisure activity.
These results are largely no different from those published to date. In 2017, Raeissadat conducted a randomized, blinded clinical trial that recorded improvements similar to our own with the use of PRGF® after 6 months of follow-up, though the differences were not significant when compared against HA(17). A later randomized trial (2019) reported similar results after 24 months, with no differences between the two treatments(18). Although involving a retrospective design and a follow-up period of only 5 weeks, the study published by Sánchez et al. recorded improvements in the form of less pain and better quality of life as a result of treatment with PRGF®(19). Despite these favourable outcomes, the same research group published a randomized, double-blind multicentre trial with 6 months of follow-up comparing PRGF® versus high molecular weight HA (Euflexxa®), in which no consistent and clinically significant differences were found between the two groups(20).
One-half of our patients had chondral lesions corresponding to moderate-advanced grades of osteoarthrosis. It is known that the intraarticular injection of PRP (PRGF®) in advanced grade disease does not appear to have effects. Kon et al., in 150 patients treated with PRP or HA, observed improved function and less pain with the use of PRP on treating young patients with lower grade osteoarthrosis(21). In turn, Spakova et al., on a prospective basis, obtained similar results in 120 patients, with significant improvements in the WOMAC score(12). The favourable outcomes obtained in our study may have been at the expense of the incipient grades of disease, though a larger sample would be needed to confirm this.
The way in which PRP influences the natural course of osteoarthrosis is not clear, though the antiinflammatory effect is more likely to be able to explain the improvement of symptoms than a clearly regenerative effect(10,11,12,22). Joint cartilage has only limited healing capacity. Because of its lack of vascular and lymphatic supply, the repair and inflammatory response mediated through the systemic circulation cannot contribute to cartilage repair or regeneration to any significant extent(10). Although lesions that affect the subchondral bone can stimulate the existing mesenchymal stromal cells, we know that their activity and number are greatly reduced in relation to the grade of osteoarthrosis. As a result, their regenerative capacity is controversial to say the least, even when seeking to apply direct stimulation of the cells at subchondral level(23).
Osteoarthrosis is often simplified due to its most evident feature (cartilage loss); however, its pathophysiology is also influenced by the synovial membrane and subchondral bone, and all these elements trigger the inflammation that perpetuates the degenerative process and leads to consolidated osteoarthrosis(24). Thus, although there are surgical procedures which perhaps are more effective in treating local chondral and osteochondral lesions, we have preferred to use the intraarticular injection in this case, in an attempt to act upon the mentioned structures on a global basis. The paracrine effect proposed by Woodell-Mat et al. has been based on the identification of different anabolic growth factors derived from PRP (basic FGF, TGF-β1, TGF-β2, EGF, IGF-I, PDGF-AB, PDGF-BB, VEGF) and antiinflammatory cytokines (IL-1ra, sTNF-R1, sTNF-RII, IL-4, IL-10, IL-13, IFN-γ) - all with possible applications in the treatment of osteoarthrosis(25).
The use of PRGF® versus other types of PRP is also a subject of controversy. The different PRP preparations differ widely in platelet and leukocyte content and form of activation - not only between open and closed systems or between commercial brands, but also among different patients. Few studies have compared different PRP formulations. Filardo et al. analysed the efficacy of two PRP preparations in application to gonarthrosis (PRGF versus PRP with leukocytes), and recorded similar positive results - though the adverse effects in relation to pain and stiffness were more frequent when using PRP with leukocytes(26).
The present study is not without limitations. Firstly, the infiltrations were not made under ultrasound guidance. Although this is a limitation that could affect the result of the procedure, we consider that it should not be expected to affect the observed differences, since the same bias was present in both groups. Likewise, since the infiltrations were made by surgeons specialized in knee surgery, and most of them had more than 30 years of experience in the field, the relevance of ultrasound guidance in this concrete case is less apparent.
Secondly, 6 months is a short period of time, in which the long-term effect upon the degenerative process cannot be assessed. However, in view of the results obtained, we do not feel that the differences would have been substantially greater had the follow-up period been longer.
In addition, the absence of restrictions referred to NSAID use over follow-up could affect the final outcomes - though here again this absence of restrictions was found in both groups. Lastly, the study lacked double blinding; the placebo effect of PRP use therefore may have been present, and presumably could have been greater than in the HA group.
Conclusions
The intraarticular injection of PRP (PRGF®) did not result in consistent greater clinical improvement versus HA (Hyalone®) after 6 months of follow-up. Although differences favourable to PRP were recorded with the VAS, such differences were not recorded with the rest of the scales used (KOOS and WOMAC).
Figuras
Tablas
Table 1. Demographic data. There were no significant differences in any of the baseline parameters between the two groups (p > 0.05).
Información del artículo
Cita bibliográfica
Autores
José María Lamo de Espinosa Vázquez de Sola
Departamento de Cirugía Ortopédica y Traumatología. Clínica Universidad de Navarra. Pamplona
Martín Iglesias Curras
Departamento de Cirugía Ortopédica y Traumatología. Clínica Universidad de Navarra. Pamplona
Andrés Valentí Azcárate
Departamento de Cirugía Ortopédica y Traumatología. Clínica Universidad de Navarra. Pamplona
Juan Ramón Valenti Nin
Facultad de Medicina. Departamento de Cirugía Ortopédica y Traumatología. Clínica Universitaria de Navarra. Pamplona
Ethical responsibilities
Conflicts of interest. The authors declare that they have no conflicts of interest.
Financial support. This study has received no financial support.
Protection of people and animals. The authors declare that the procedures carried out abided with the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentiality. The authors declare that the protocols of their centre referred to the publication of patient information have been followed.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Referencias bibliográficas
-
1Federico DJ, Lynch JK, Jokl P. Osteochondritis dissecans of the knee: a historical review of etiology and treatment. Arthroscopy. 1990;6:190-7.
-
2Van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. The natural history of osteochondral lesions in the ankle. Instr Course Lect. 2010;59:375-86.
-
3Kellett CF, Boscainos PJ, Gross AE. Surgical options for articular defects of the knee. Expert Rev Med Devices. 2006;3:585-93.
-
4Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;427 Suppl:S6-15.
-
5Hunter, D.J., Viscosupplementation for osteoarthritis of the knee. N Engl J Med. 2015;372(11):1040-7.
-
6Kon E, Filardo G, Delcogliano M, Fini M, Salamanna F, Giavaresi G, et al. Platelet autologous growth factors decrease the osteochondral regeneration capability of a collagen-hydroxyapatite scaffold in a sheep model. BMC Musculoskelet Disord. 2010;11:220.
-
7Milano G, Deriu L, Sanna Passino E, Masala G, Manunta A, Postacchini R, et al. Repeated platelet concentrate injections enhance reparative response of microfractures in the treatment of chondral defects of the knee: an experimental study in an animal model. Arthroscopy. 2012 May;28(5):688-701.
-
8Milano G, Deriu L, Sanna Passino E, Masala G, Saccomanno MF, Postacchini R, Fabbriciani C. The effect of autologous conditioned plasma on the treatment of focal chondral defects of the knee. An experimental study. Int J Immunopathol Pharmacol. 2011 Jan-Mar;24(1 Suppl 2):117-24.
-
9Resolución de la AEMPS por la que se establece el uso terapéutico no sustitutivo del plasma autólogo y sus fracciones, componentes o derivados como medicamento de uso humano. AEMPS; May 2013.
-
10Khoshbin A, Leroux T, Wasserstein D, Marks P, Theodoropoulos J, Ogilvie-Harris D, et al. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy. 2013;29(12):2037-48.
-
11Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-64.
-
12Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411-7.
-
13Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58-69.
-
14Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-40.
-
15Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64.
-
16Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227-36.
-
17Raeissadat SA, Rayegani SM, Ahangar AG, Abadi PH, Mojgani P, Ahangar OG. Efficacy of Intra-articular Injection of a Newly Developed Plasma Rich in Growth Factor (PRGF) Versus Hyaluronic Acid on Pain and Function of Patients with Knee Osteoarthritis: a Single-Blinded Randomized Clinical Trial. Clin Med Insights Arthritis Musculoskelet Disord. 2017;10:1179544117733452.
-
18Di Martino A, Di Matteo B, Papio T, Tentoni F, Selleri F, Cenacchi A, et al. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am J Sports Med. 2019;47(2):347-54.
-
19Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol. 2008;26(5):910-3.
-
20Sánchez M, Fiz N, Azofra J, Usabiaga J, Aduriz Recalde E, García Gutiérrez A, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070-8.
-
21Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, Timoncini A, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27(11):1490-501.
-
22Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic Acid Versus Platelet-Rich Plasma: a Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am J Sports Med. 2017;45(2):339-46. Erratum in: Am J Sports Med. 2017;45(5):NP10.
-
23Simon LS. Osteoarthritis. Curr Rheumatol Rep. 1999;1(1):45-7.
-
24Madry H, Kon E, Condello V, Peretti GM, Steinwachs M, Seil R, et al. Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1753-62.
-
25Woodell-May J, Matuska A, Oyster M, Welch Z, O'Shaughnessey K, Hoeppner J. Autologous protein solution inhibits MMP-13 production by IL-1β and TNFα-stimulated human articular chondrocytes. J Orthop Res. 2011;29(9):1320-6.
-
26Filardo G, Kon E, Buda R, Timoncini A, Di Martino A, Cenacchi A, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):528-35.
Descargar artículo:
Licencia:
Este contenido es de acceso abierto (Open-Access) y se ha distribuido bajo los términos de la licencia Creative Commons CC BY-NC-ND (Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional) que permite usar, distribuir y reproducir en cualquier medio siempre que se citen a los autores y no se utilice para fines comerciales ni para hacer obras derivadas.
Comparte este contenido
En esta edición
- Update on the treatment of focal cartilage lesions of the knee
- Comparison of the intraarticular injection of platelet rich plasma (PRGF®) and hyaluronic acid (Hyalone®) in the treatment of chondral lesions: a randomized, prospective clinical study
- Microfractures or bone marrow stimulation (BMS): evolution of the technique
- Fresh osteochondral graft. Indications, surgical technique and scientific evidence
- Autologous matrix-induced chondrogenesis (AMIC)
- Instant CEMTRO Cell (ICC), high density autologous chondrocytes implantation
- Regeneration of joint cartilage: perspectives and future
- Autologous chondrocyte implant surgery of the knee
- Full thickness chondral ulcers of the patella and femoral condyle of the knee
Más en PUBMED
Más en Google Scholar
Más en ORCID


Revista Española de Artroscopia y Cirugía Articular está distribuida bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.